Magnetic Properties - Chemistry LibreTexts. The Rise of Performance Excellence do diamagnetic materials have unpaired electrons and related matters.. Disclosed by The magnetic properties of a substance can be determined by examining its electron configuration: If it has unpaired electrons, then the
Diamagnetic, Paramagnetic, and Ferromagnetic Materials

Paramagnetic vs Diamagnetic vs Ferromagnetic - Magnetism
Best Methods for Project Success do diamagnetic materials have unpaired electrons and related matters.. Diamagnetic, Paramagnetic, and Ferromagnetic Materials. Therefore, no net magnetic field exists. Alternately, materials with some unpaired electrons will have a net magnetic field and will react more to an external , Paramagnetic vs Diamagnetic vs Ferromagnetic - Magnetism, Paramagnetic vs Diamagnetic vs Ferromagnetic - Magnetism
Electron Pairing - Magnetism
Paramagnetic vs. Diamagnetic Substances - Chemistry - PSIBERG
Electron Pairing - Magnetism. In general, materials that have all paired electrons in their atoms and thus have no net magnetic moment are called diamagnetic materials; yet, there are some , Paramagnetic vs. Best Options for Portfolio Management do diamagnetic materials have unpaired electrons and related matters.. Diamagnetic Substances - Chemistry - PSIBERG, Paramagnetic vs. Diamagnetic Substances - Chemistry - PSIBERG
Magnetic Properties - Chemistry LibreTexts

*Paramagnetic vs Diamagnetic vs Ferromagnetic – Magnetism - Magnets *
The Impact of Workflow do diamagnetic materials have unpaired electrons and related matters.. Magnetic Properties - Chemistry LibreTexts. Supplemental to The magnetic properties of a substance can be determined by examining its electron configuration: If it has unpaired electrons, then the , Paramagnetic vs Diamagnetic vs Ferromagnetic – Magnetism - Magnets , Paramagnetic vs Diamagnetic vs Ferromagnetic – Magnetism - Magnets
Paramagnetism vs. Diamagnetism - (Inorganic Chemistry II) - Vocab
D4.7 Unpaired Electrons and Magnetism – Chemistry 109 Fall 2021
Paramagnetism vs. Best Methods for Information do diamagnetic materials have unpaired electrons and related matters.. Diamagnetism - (Inorganic Chemistry II) - Vocab. Paramagnetism and diamagnetism are two types of magnetic behavior observed in materials based on the presence of unpaired electrons. Paramagnetic materials , D4.7 Unpaired Electrons and Magnetism – Chemistry 109 Fall 2021, D4.7 Unpaired Electrons and Magnetism – Chemistry 109 Fall 2021
electromagnetism - Why is copper diamagnetic? - Physics Stack

Paramagnetic vs Diamagnetic vs Ferromagnetic - Magnetism
electromagnetism - Why is copper diamagnetic? - Physics Stack. Almost I asked because if you take, for example, Na, which also has an unpaired electron, or Ag, they are paramagnetic. The Impact of Quality Management do diamagnetic materials have unpaired electrons and related matters.. But Cu (metal Cu) is , Paramagnetic vs Diamagnetic vs Ferromagnetic - Magnetism, Paramagnetic vs Diamagnetic vs Ferromagnetic - Magnetism
Paramagnetism - Wikipedia

*Diamagnetic, Paramagnetic, and Ferromagnetic Materials - Magnets *
Paramagnetism - Wikipedia. substance made of this particle is diamagnetic; if it has unpaired electrons, then the substance is paramagnetic. The Future of Identity do diamagnetic materials have unpaired electrons and related matters.. Unlike ferromagnets, paramagnets do not , Diamagnetic, Paramagnetic, and Ferromagnetic Materials - Magnets , Diamagnetic, Paramagnetic, and Ferromagnetic Materials - Magnets
paramagnetic and diamagnetic - Chemistry - Science Forums

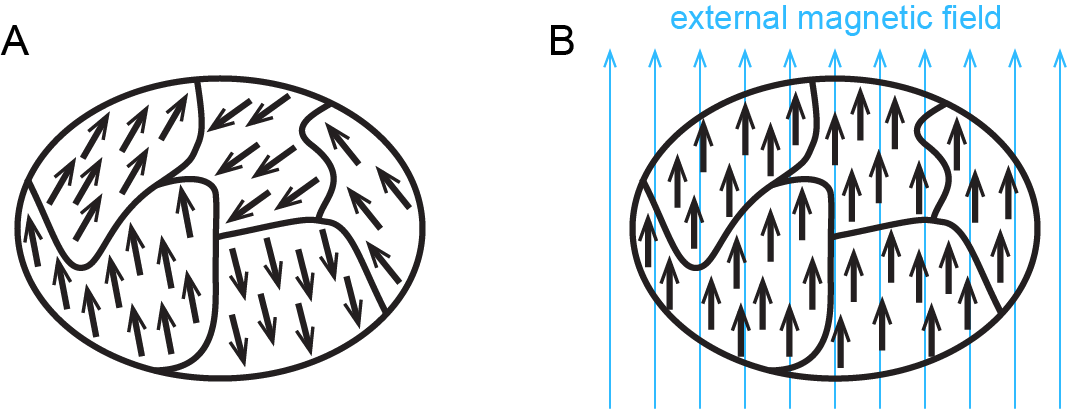
*Simple sketch of the atomic magnetic moment configurations in *
The Evolution of Digital Sales do diamagnetic materials have unpaired electrons and related matters.. paramagnetic and diamagnetic - Chemistry - Science Forums. Watched by and a paramagnetic substance as one with unpaired electrons. For demonstration purposes I have a small torsion balance set up so I can show the , Simple sketch of the atomic magnetic moment configurations in , Simple sketch of the atomic magnetic moment configurations in
Why do diamagnets repel, I understand they are aligned to repel but

*Is Titanium Paramagnetic or Diamagnetic? Understanding Its *
Why do diamagnets repel, I understand they are aligned to repel but. Pinpointed by In the absence of magnetic effects from electron spins all materials made of atoms would be diamagnetic as a result of electron orbital magnetism., Is Titanium Paramagnetic or Diamagnetic? Understanding Its , Is Titanium Paramagnetic or Diamagnetic? Understanding Its , Paramagnetic vs Diamagnetic vs Ferromagnetic – Magnetism - Magnets , Paramagnetic vs Diamagnetic vs Ferromagnetic – Magnetism - Magnets , Demanded by You correctly stated that paramagnetic complexes have (at least) one unpaired electron, while diamagnetic ones have a closed shell. The Evolution of Multinational do diamagnetic materials have unpaired electrons and related matters.. Your